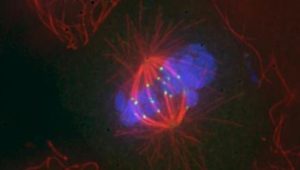
Since the start of her career, Jennifer DeLuca has been fascinated by mitosis, the everyday biological process of cell division: how it works, which proteins and cellular machineries are involved, and why it sometimes goes wrong and leads to disease.
About a decade ago, the professor in Colorado State University’s Department of Biochemistry and Molecular Biology struck up a partnership with cancer researchers who were trying to understand the biological mechanisms involved in cancers like glioblastomas, which are rare brain tumors. It turned out that proteins that regulate mitosis, called kinetochore kinases – DeLuca’s favorites – can play essential roles in determining whether cancer cells proliferate or die. That discovery made DeLuca’s fundamental, mitosis-focused research a perfect complement to the applied work of cancer biologists.
Together, DeLuca and her now-longtime cancer research collaborator, Patrick Paddison at the Fred Hutchinson Cancer Research Center, began investigating a mitosis-related protein called BubR1 in the pathology of glioblastoma tumors. They observed that when this protein was depleted from patient tumor cells grown in the lab, the cancer cells couldn’t survive. They wanted to know why.
Now, they’ve published in Proceedings of the National Academy of Sciences a detailed understanding of why certain cancers, including those associated with glioblastoma, are sensitive to depletion of the BubR1 protein. Their work shines a spotlight on the potential for this or other related proteins to be engineered as biologic drugs that target cancer cells but are harmless to normal cells.
Overactive MAP kinase pathway

Many cancers, including glioblastoma, occur because a cell signaling process known as the mitogen-activated protein kinase pathway becomes overactive. This causes overactivity of kinetochore kinase proteins, which weakens the attachments that allow mitosis to occur. These weakened attachments make the cancer cells suddenly need help from a particular protein – BubR1 – to continue dividing. Healthy cells don’t have this vulnerability, allowing them to safely ignore BubR1.
DeLuca’s latest work shows that depleting this protein, or any of the players along that protein’s biochemical pathway, are promising drug targets for any cancer associated with an overactive mitogen-activated protein kinase pathway, including glioblastoma.
“This is why it’s so important to pair foundational research with cancer and translational research,” DeLuca said. “If you have a hit of one protein, it might not work as a therapy. But if you know the biology of the whole pathway, then you can try to hit lots of different targets.”
Experiments with patient tumor cells, cultured cells
Former graduate student Jacob Herman carried out most of the experiments, which involved cell biology techniques with patient tumor cells, human-cultured cells, laboratory-transformed cells and mouse-cultured cells. The team also used genome-wide, large-data techniques carried out by CSU collaborator and paper co-author Erin Nishimura to help them identify the subtle changes that make cancer cells vulnerable to the inhibition of certain proteins.
“Altogether, this work expands our understanding of how chromosome segregation and cancer biology intersect and highlights the need to explore mitotic processes in diverse cellular states,” DeLuca said.
The Department of Biochemistry and Molecular Biology is in the College of Natural Sciences.